Sodium Carbonate Decomposes to Produce Sodium Oxide and Carbon Dioxide
The reaction takes place in a boiling solution. 2 N a H C O 3 s Δ N a 2 C O 3 2 s C O 2 g H 2 O l.

Na2co3 Hcl Sodium Carbonate Hydrochloric Acid Youtube
A sample of 5873 mL gas was collected using water displacement method at 27 C at a total pressure of 760 mm Hg and vapour pressure of water is 28 mm Hg.

. Na2CO3 s Na2O s CO2 g The name of the first. When a single compound breaks down into two or more compounds or elements in a chemical reaction then it is known as decomposition reaction. A calculate the mass of carbon oxide collected.
Using the balanced chemical equation from 1 predict how many grams of sodium carbonate are produced theoretical yield when 250g of sodium bicarbonate decompose. Nitrogen oxide gas reacts with carbon monoxide gas to produce nitrogen gas and carbon diavida con This problem has been solved. Sodium and water are combined to produce sodium hydroxide and hydrogen.
Na2CO3 s Na2O s CO2 g A. This type of reaction is called a thermal decomposition. Yes it isSodium Carbonate Sodium Oxide Carbon Dioxide NA2CO3 â Na2O CO2.
The second potential explanation is that the sodium bicarbonate decomposes into sodium carbonate Na 2 CO 3 carbon dioxide CO 2 and water when it is heated. This takes place above 500 degree C. The chemical symbol for sodium carbonate is.
The balanced equation for the decomposition of sodium bicarbonate into sodium carbonate carbon dioxide and water is. The decomposition of sodium bicarbonate will result in the formation of sodium oxide and carbon dioxide. When heated at high temperature sodium carbonate Na2CO3decomposes to sodium oxide and carbon dioxide gas.
When it is heated it undergoes a thermal decomposition reaction. Solid aluminum carbonate decomposes to form solid aluminum oxide and carbon dioxide gas. Is Al2CO33 Al2O3 CO2 the right equation.
When heated sodium bicarbonate NaHCO3 decomposes into sodium carbonate Na2CO3 water and carbon dioxide. When heated sodium bicarbonate NaHCO3 decomposes into sodium carbonate Na2CO3 water and carbon dioxide. When sodium carbonate is heated then its decomposes into sodium oxide and carbon dioxide.
What does sodium carbonate decompose into when heated. When it is heated above about 80C it begins to break down forming sodium carbonate water and carbon dioxide. Solid sodium carbonate decomposes to produce solid sodium oxide and carbon dioxide gas.
When carbon is burned in air it reacts with oxygen to form carbon dioxide. Sodium Carbonate and Hydrochloric Acid Reaction Na 2 CO 3 HCl. Hence carbon dioxide will produce with sodium oxide on decomposition of.
Write a balanced chemical equation for this reaction. 2 NaHCO3 s Na2CO3 s CO2 g H2O g Like most chemical reactions the rate of the reaction depends on temperature. Sodium hydrogen carbonate also known as sodium bicarbonate or bicarbonate of soda has the chemical formula NaHCO3.
When sodium carbonate is heated then its decomposes into sodium oxide and carbon dioxide. Solid sodium carbonate decomposes to produce solid sodium oxide and carbon dioxide gas Write a balance equation using the correct formulas and include conditions. Solid sodium carbonate is heated to give solid sodium oxide and.
Your email address will not be published. The decomposition of sodium carbonate is. When calcium carbonate is heated it decomposes into calcium oxide and carbon dioxide gas.
Sodium carbonate decomposes to sodium oxide and carbon dioxide. 4Cu O2 2Cu2O. Nitrogen oxide gas reacts with carbon monoxide gas to produce nitrogen gas and carbon dioxide gas.
The reaction of decomposition is. While sodium carbonate is fairly stable it slowly decomposes in dry air to form sodium oxide and carbon dioxide. In Activity 1 solid sodium bicarbonate is heated and decomposes into water vapor carbon dioxide gas and solid sodium carbonate.
At temperatures above 176 degrees Fahrenheit 80 degrees Celsius sodium bicarbonate begins to form three compounds including sodium carbonate NaCO water HO and carbon dioxide CO. Sodium carbonate decomposes to produce sodium oxide and carbon dioxide. Sodium carbonate decomposes to produce sodium oxide and carbon dioxide.
Sodium bicarbonate decomposes to give sodium carbonate carbon dioxide and water. It is known that when a single compound breaks down into two or more different substances then this type of reaction is known as decomposition reaction. What is the balanced equation.
Leave a Reply Cancel reply. The decomposition of sodium carbonate is. If 500 g of NaHCO3 decomposes what is the mass of the carbon dioxide produced.
The reaction is balanced as NaOH CuSO4 CuOH2 Na2SO4. The balanced chemical equation for this reaction is 2NaHCO 3 s Na 2 CO 3 s CO 2 g H 2 O g The third potential explanation is that the sodium bicarbonate decomposes into sodium oxide Na 2 O. The Balanced Equation.
The decomposition of sodium bicarbonate will result in the formation of sodium oxide and carbon dioxide. Sodium carbonate Na2CO3 or CNa2O3 CID 10340 - structure chemical names physical and chemical properties classification patents literature biological. Sodium hydroxide - diluted solution.
Sodium bicarbonate NaHCO also known as baking soda is a chemical that can undergo decomposition when heated. Required fields are marked Comment. If 500 g of NaHCO3 decomposes what is the mass of the carbon dioxide produced.
Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. The decomposition reaction can be accelerated by heating the washing soda to 851 C 1124 K. When aqueous hydrochloric acid is added to aqueous sodium carbonate Na 2 CO 3 solution carbon dioxide CO 2 gas sodium chloride NaCl ad water are given as productsAlso HCl can be added to solid Na 2 CO 3We will discuss about different characteristics of sodium carbonate and HCl acid reaction in.
When sodium carbonate is heated show the reaction. The chemical symbol for sodium carbonate is. It is known that when a single compound breaks down into two or more different substances then this type of reaction is known as decomposition reaction.

Discuss The Various Reactions That Occur In The Solvay Process

How To Balance Na2co3 Na2o Co2 Sodium Carbonate Decomposing At High Temp Youtube
How Can We Produce Sodium Bicarbonate Using Sodium Carbonate Quora

Word And Balanced Formula Equations

Calculate The Volume Measured At S T P Of Carbon Dioxide Released When 8 40 G Of Sodium Hydrogen Carbonate Is Decomposed According To The Equation 2nahco 3 Tona 2 Co 3 H 2 O Co 2 Na 23 H

Where Find Sodium Tin Iv Oxide Hydrate Na2sno3 Xh2o Crystalline Inorganic Compound Sodium Hydration

How To Balance Naoh Co2 Na2co3 H2o Sodium Hydroxide Plus Carbon Dioxide Youtube

Sodium Carbonate Na2co3 Pubchem
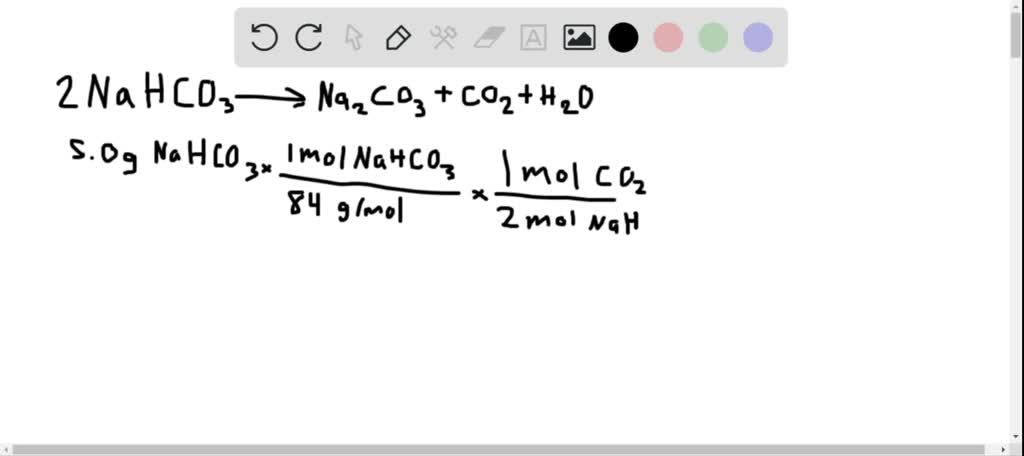
Solved Sodium Bicarbonate Nahco Is Called Baking Soda Because When Heated It Releases Carbon Dioxide Gas Which Is Responsible For The Rising Of Cookies Some Doughnuts And Cakes A Calculate The Volume In

Sodium Carbonate Hydrochloric Acid Na2co3 Hcl Molecular Equations Net Ionic Equations Youtube

Washing Soda Video Khan Academy

Equation For Sodium Carbonate Dissolving In Water Na2co3 H2o Youtube
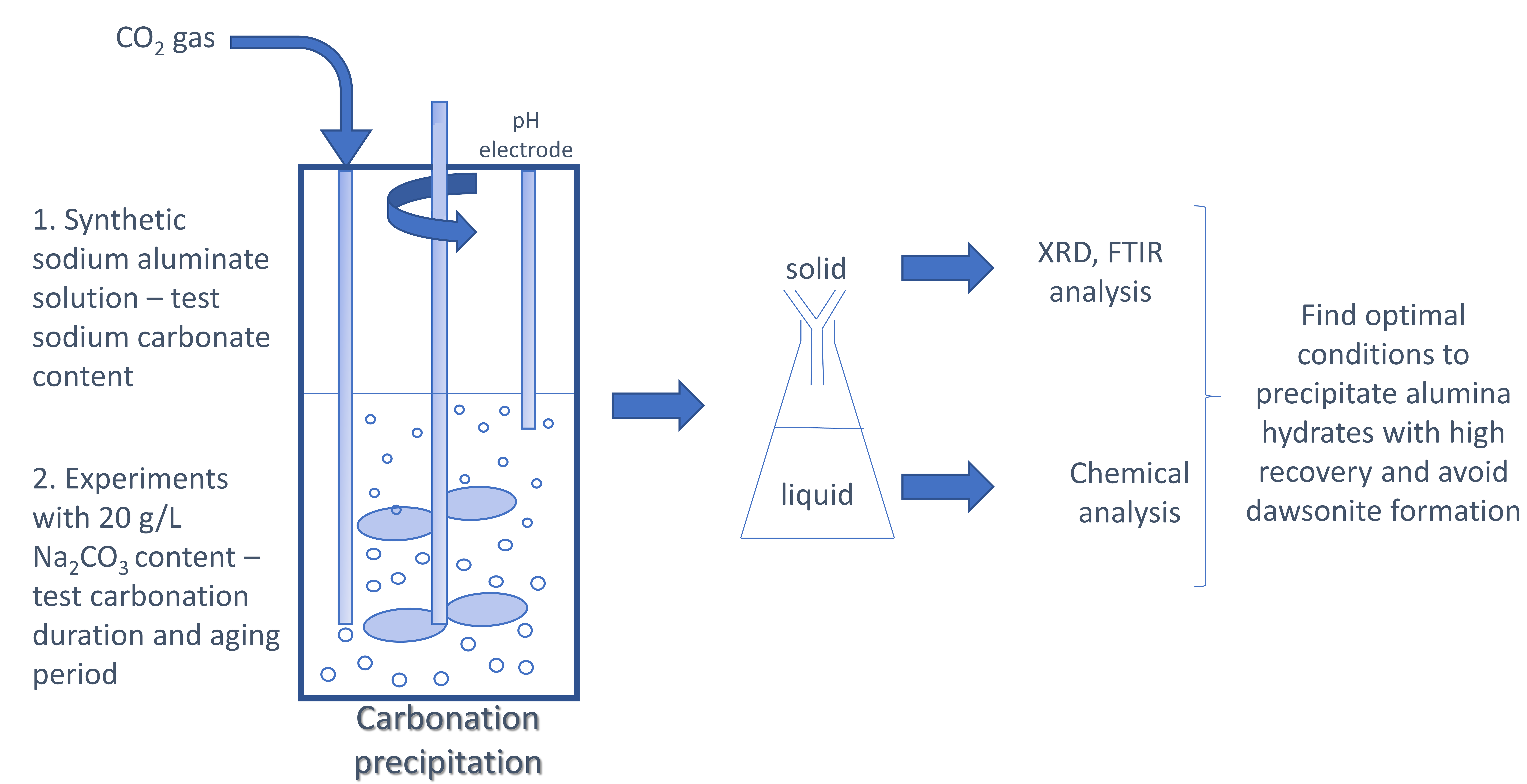
Crystals Free Full Text Carbonation Of Sodium Aluminate Sodium Carbonate Solutions For Precipitation Of Alumina Hydrates Avoiding Dawsonite Formation Html
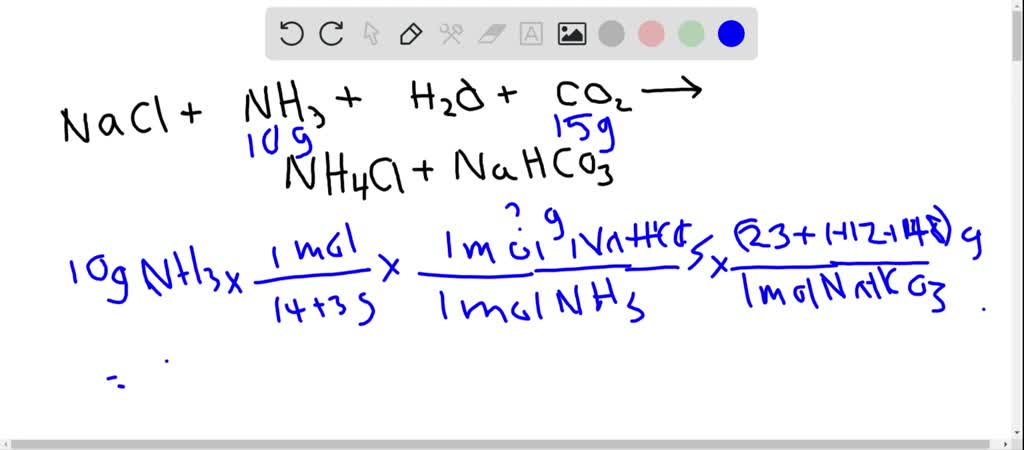
Solved One Process For The Commercial Production Of Baking Soda Sodium Hydrogen Carbonate Involves The Following Reaction In Which The Carbon Dioxide Is Used In Its Solid Form Dry Ice Both To Serve

Type Of Reaction For Nahco3 Na2co3 H2o Co2 Youtube

Pdf Carbon Dioxide Conversion Into The Reaction Intermediate Sodium Formate For The Synthesis Of Formic Acid

Washing Soda Property Benefits And Preparation Of Sodium Carbonate

Sodium Carbonate Hydrochloric Acid Na2co3 Hcl Molecular Equations Net Ionic Equations Youtube

How To Balance Na2co3 Co2 H2o Nahco3 Sodium Carbonate Carbon Dioxide Water Youtube
Comments
Post a Comment